Koneksa, the global leader in digital biomarker development, is empowering researchers with a robust respiratory solution that delivers validated endpoints from any location. We understand that obtaining the precise and complete data needed to paint a holistic, ecologically valid picture of respiratory patient health for accurate treatment effect detection can be difficult. Factors like diurnal variability, inaccurate devices, incorrectly performed participant maneuvers, large sample size requirements, and travel burdens are significant challenges in clinical trials.
Koneksa solves all these problems.

We are a science-driven one-stop shop for your respiratory clinical trial needs
Our leading-edge cloud-based platform, innovative algorithms, and suite of top-of-the-line integrated devices provide precisely the data your studies need. We support measures from ultrasonic spirometry, ePROs, actigraphy, and pulse oximetry through our device-agnostic platform, so you can confidently collect the measures that matter for your study — around the clock and around the world.
We believe that a truly comprehensive data set collected as patients go about their daily lives is necessary to properly evaluate a treatment. By partnering with us at the earliest stage of development of your clinical trial, you can rest easy knowing that our highly configurable solution is customized to deliver clearer insights and help you make confident go/no-go decisions earlier, with fewer participants, potentially saving months and millions of dollars.
Multiple solutions from multiple sources complicate your journey from development to market. Koneksa fixes that. We offer a complete respiratory solution built for your study and inspired by your patients, starting with an ultrasonic spirometer.
Our turnkey spirometry toolkit includes:
- A provisioned iPhone with the Koneksa mobile app
- A Bluetooth-enabled ultrasonic spirometer
- Detachable hygienic filters
- Nose clips
- An easy-to-follow patient guide
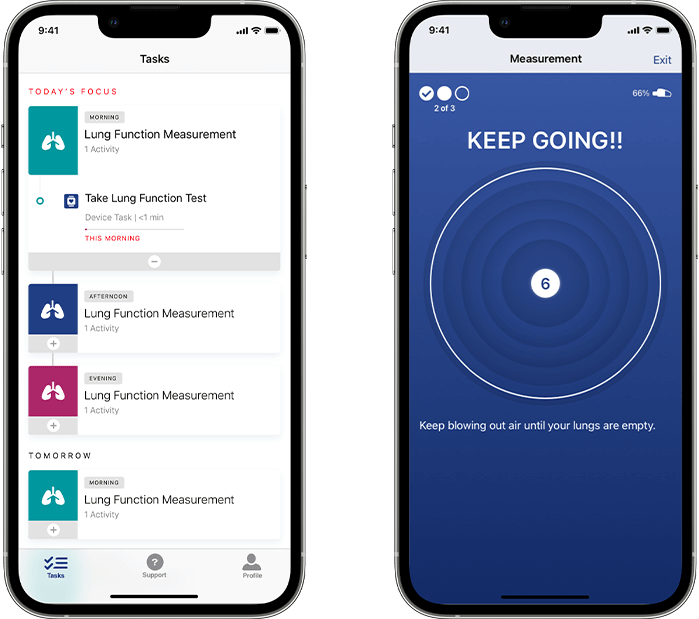
Our respiratory solution offers configurable scheduling, assessment reminders, and in-app audio and on-screen coaching based on the latest guidelines from the American Thoracic Society (ATS) and European Respiratory Society (ERS). Participants experience the Koneksa mobile app in their native language and dialect, allowing us to support clinical studies with global reach. Spirometry maneuvers are supported through real-time quality control to collect high-quality measurements.
Once assessments are completed, configured endpoints are automatically and instantly transmitted from the phone to Koneksa’s SaaS platform, where they can be viewed alongside the full flow volume loops and monitored for quality and compliance.
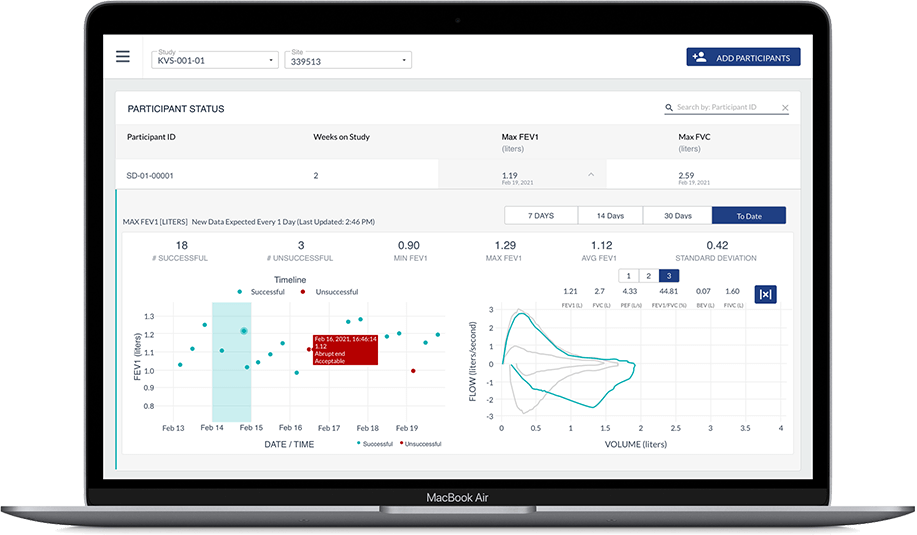
Our rigorous, science-first approach has been successfully deployed in numerous patient populations:
Allergy
Asthma
COPD
COVID-19
Interstitial lung disease
With 24/7 end-to-end support that extends from study design consulting all the way to global regulatory assistance, Koneksa is your first and last stop for digital respiratory measurements.
Faster results for earlier decisions
Our highly configurable system allows you to collect more data faster than would be possible for studies utilizing only in-clinic measures. Flexible scheduling with alerts and reminders lets you define assessment timing and the overall rate of data collection. With more data points in a shorter period, your respiratory study gets done faster without sacrificing quality — better for you, better for patients.
Koneksa’s respiratory solution also provides the benefits of repeated measures. By capturing a high volume of data, you can control for factors that drive variation in spirometry measures, increasing your study’s statistical power and decreasing the number of patients needed to demonstrate treatment effect. We also have the experience in this area to help you confidently work through the complications of diurnal and seasonal variability that are all too common to respiratory measures. What’s more, you have access to all of your study’s measures immediately through our cloud-based platform, allowing you to stay on top of patient health and make timely, crucial decisions.

Ultrasonic precision for in-clinic quality
Koneksa’s high-quality respiratory data are obtained through technology with capabilities that far exceed traditional turbine-based spirometers. The greater sensitivity of an ultrasonic spirometer provides precise readings even for patients with low lung function, so you can be confident in the accuracy of your respiratory data. Ultrasonic technology is even more powerful when FVC is a required measure, a common focus when researching interstitial lung disease (e.g., idiopathic pulmonary fibrosis). With our solution, you can collect and compare inhalation and exhalation data, ensuring ATS compliance for each successful maneuver and providing consistent, quality data for your trial’s endpoints.
Measures include:
- FEV1
- FVC
- PEF
- FEV1/FVC
- BEV
- FIVC
Our Bluetooth ultrasonic spirometer is also drastically superior to turbine devices because there are no moving parts. While turbines depend on spinning plastic pieces, require frequent calibration, and can develop obstructions that impact turbine movement, ultrasonic units avoid these limitations. They are also easier to clean and much less likely to become contaminated.
In short, ultrasonic spirometers are more precise, easier to use, and easier to maintain.
Greater patient convenience for more frequent data collection
Patient convenience matters. That’s why our science-driven approach creates the best possible patient experience while eliminating geographical and mobility constraints. Koneksa’s respiratory solution can be used at home, brought to the clinic, or used during home visits, eliminating travel-related patient burdens and allowing for high-frequency data collection. Spirometry measures and configurable questionnaires can be completed within the same repeatable mobile experience, improving ease of use and participant compliance while providing you with additional data.
Patients deserve access to novel therapies for their respiratory conditions, and we provide sponsors with a respiratory solution that delivers on that objective — around the clock and around the globe.

