Rare diseases pose unique challenges to research
The combination of small and dispersed populations, a translation gap, and heterogeneity within and between subjects combine to challenge the development of novel therapies.
Sponsors need the tools to illuminate poorly understood diseases, support endpoints, and enable remote data collection to reduce patient burden, despite limited patient numbers. Koneksa’s digital biomarker solutions address that need and open the door to previously inaccessible insights into rare diseases.

Koneksa’s rare disease experience includes a variety of areas, and our internal R&D efforts also include a rare disease focus.
Relevant measures:
- Activity
- Sleep
- Gait
- Vision
- Speech
- Cognitive
- Pain
- Seizures
- Vital signs
As the industry leader in digital biomarkers, we can design and develop configurable digital biomarkers that help you answer your most critical questions. Our measurement tools are remotely monitored for operational integrity and patient compliance at home. Plus, with multilingual capabilities and a global footprint, our solution empowers you to connect with difficult-to-access patients wherever they are, thus easing not only patient burden, but also the burden of rare disease caregivers.
Think beyond your current endpoints to what digital technology can unleash.
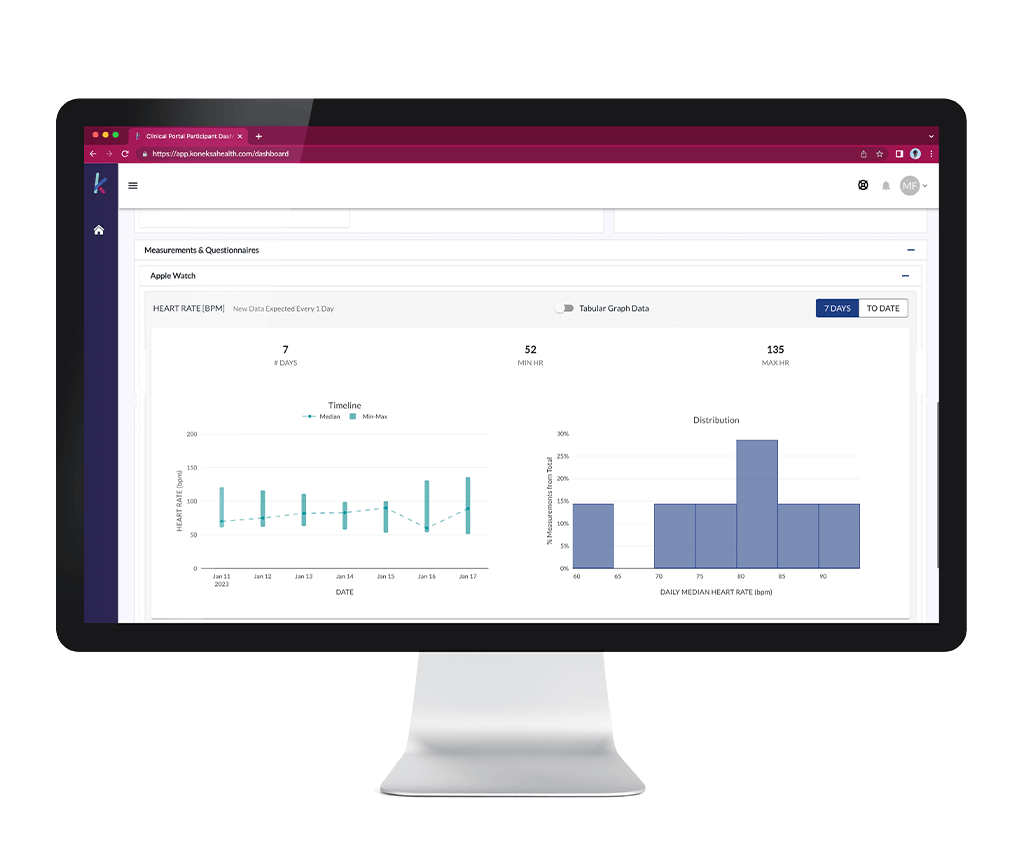
Our Platform Is Your Window for Valuable Insight
Not only do Koneksa’s solutions enable global study execution, but they also help you understand disease heterogeneity by making it easy to collect multiple measurements, covering a broad array of signs and symptoms.
Given the difficulty in finding and enrolling rare disease patients, it is imperative that sponsors, patients, and caregivers equally capitalize on the opportunity to conveniently collect as much relevant data as possible. By recording high-volume/high-resolution data from multiple sources into our cloud-based platform in real time, you get a constantly updated and informative window into rare disease patients’ study compliance and can be confident that the data collected is sufficient to answer your scientific questions. That’s how we help you, and that’s how we improve your ability to help patients.
We Are the Leaders in Developing Evidence-Backed Rare Disease Measurement Concepts
Koneksa is dedicated to helping all our rare disease partners go through the journey of identifying, developing, and validating meaningful measures. We recognize that what is meaningful to patients and clinicians may overlap in some ways and differ in others. That is why a shared understanding of the biomarker objective is important.
We routinely support patient working groups, expert review panels, and pilots to advance measurement concept development. Rare disease patients are, by definition, few in number, so ensuring you are collecting truly informative data is essential to your program and to patients. By collecting meaningful data in the context of patients’ daily lives — and more of it — you gain the sharp insights needed to accurately assess treatment effect.

Deep Experience, Broad Range
Through a flexible, innovative mindset and experience in a wide array of diseases and indications, we have developed the finely honed technical expertise needed to configure the right measures for your rare disease trial.
Our work includes 70+ standard measures deployed to 1,000+ global sites in 20+ disease areas. Our therapeutic experience includes glucose transporter type 1 deficiency syndrome (Glut1DS), hemophilia, Angelman syndrome, achondroplasia, Fabry disease, sickle cell disease, paroxysmal nocturnal hemoglobinuria (PNH), and ataxia. With our experience and technology, we can help you bring much-needed treatments to patient populations who sorely need them.
We Hear the Rare Disease Patient’s Voice
We engage with patients and caregivers to better understand the challenges of their symptoms and needs, leading to better solutions for sponsors and a better trial experience for all stakeholders. Trials with new technologies simply work better when patients and caregivers join the design process, and we are constantly humbled by the insights patients provide.
Koneksa proposes methods to patients and asks key questions, such as:
Are the measures this device collects meaningful to you in terms of your experience with the disease and its impact on your daily life?
What do you think about the usability of these candidate devices?
How often could you routinely record data on the signs and symptoms you experience?
With that information, we help design your protocol and overall development and validation approach. We then support the execution of your trial with end-to-end services that include data analysis and regulatory submissions.
Our team works with you every step of the way to put data collection in patients’ hands. This eliminates travel and reduces patient burden, while giving you real-time access to your trial’s measures so you can stay on top of patient health status.
No matter where your patients are, the data you need are always at hand.
Koneksa Delivers at a Higher Level; We Deliver What Matters
Koneksa helps solve the challenges of rare disease trials with at-home digital measurements and biomarkers, developed and deployed in partnership with patients, caregivers, experts, and sponsors. In rare diseases, the gold standard is typically a patient reported outcome (PRO), often adapted from another primary purpose. But there is room for something much more robust than this. You need better measures that support endpoints that can progress your program more efficiently.
You need something that is more beneficial to rare disease patients.